
Which Covid Vaccine Completed Phase 3. Letter from Dr Nikki Kanani and Caroline Temmink announcing publication of the Enhanced Service Specification for phase 3 of the COVID-19 vaccination programme and guidance on the General Practice Phase 3 Expression of Interest and Site Designation process. Notably this has not been described in current reports of SARS-CoV-2 vaccine phase 3 trials. Qatar Cabinet announced that the phase 3 of the gradual lifting of Covid-19 restrictions will go into effect from Friday. On 12 April the DCGI approved Russias Sputnik V vaccine for emergency use in India.
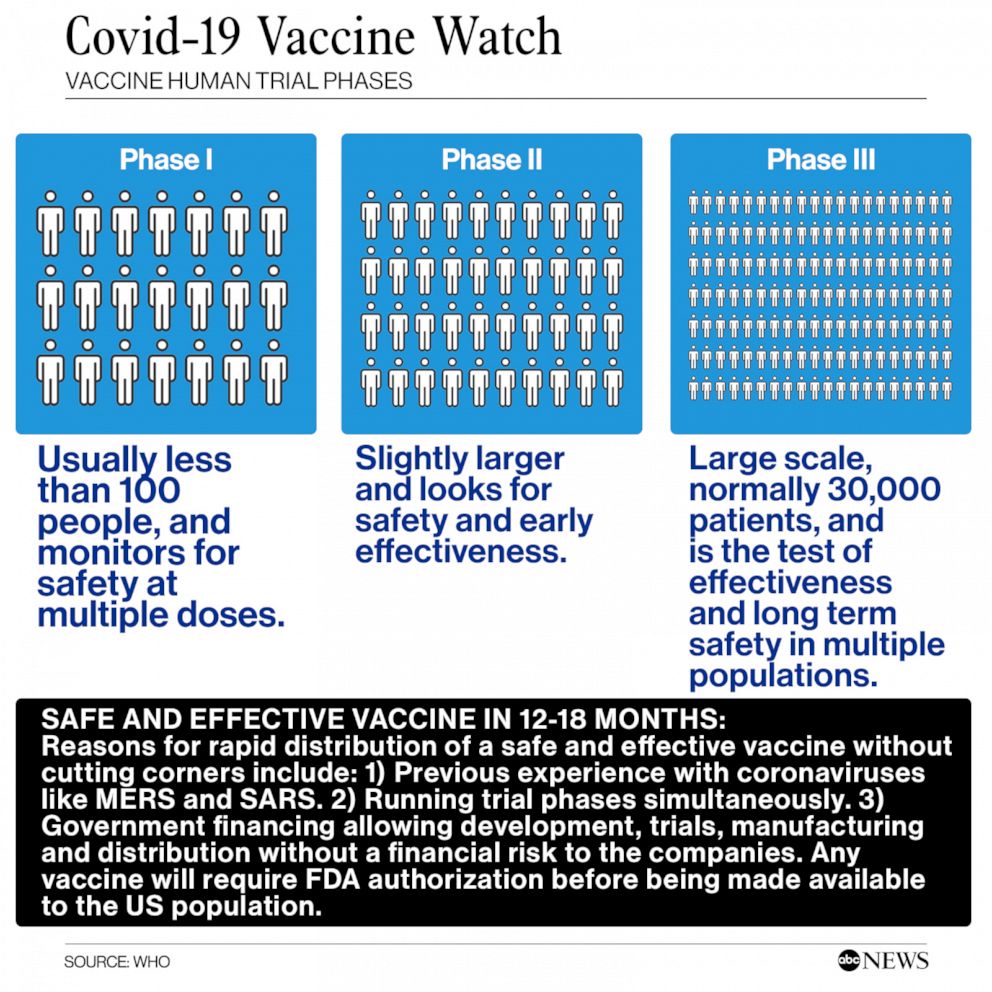
Published 14 July 2021. Seven COVID-19 vaccines in the third phase of clinical trials. COVID-19 Vaccines Approved for Emergency Use. CLAIM3- None have completed initial researchtrials All four vaccines given emergency authorization in the US. DCGI Clears Indias Indigenous MRNA Vaccine For Phase 23 Clinical Trials Under Indias COVID-19 vaccination expansion strategy the Drug Controller General of India DCGI on Tuesday gave approval to Indias first mRNA based vaccine. Reddys Laboratories stated that it planned to.
Type of vaccine.
170 confirmed cases of COVID-19 were evaluated with 162 observed in the placebo group versus 8 in. On 12 April the DCGI approved Russias Sputnik V vaccine for emergency use in India. Phase 3 clinical trial of COVID-19 vaccine underway August 6 2020 As the race to develop a safe and effective vaccine to protect against COVID-19 continues phase 3 trials of investigational vaccines are underway. The table belows shows COVID-19 vaccines that have been approved for emergency use green have reported vaccine efficacy from a Phase 3 trial blue or are under investigation in a Phase 3 trial. MRNA Phase 3 status. Type of vaccine.