
What Types Of Covid 19 Vaccines Are In Clinical Trials. 95 CI 059 to 083. Once sufficient knowledge on the immunogenicity response to COVID-19 vaccines is acquired non-inferiority immunogenicity trialscomparing the immune response of a vaccine candidate to that of an authorised vaccinewould probably be the most common trial design. COVID-19 vaccine clinical trials including vaccines in earlier stages of development by visiting clinicaltrialsgov. 89 rows Immunogenicity and adverse events following immunization with alternate schedules of.
Only in phase III trials is vaccine efficacy assessed. Different types of immune responses are often measured including antibodies and cell-mediated immunity but phase II trials do not assess how well a vaccine actually works. Absolute risk reduction 07. Based on data reported by the manufacturer for PfzierBioNTech vaccine BNT162b2 this critical appraisal shows. Vaccine efficacy and safety have not been reported in pregnant women in any clinical trials for any of the vaccine candidates. 104 rows The FDA announced revisions to the patient and provider fact sheets for the.
Information about the JJJanssen COVID-19 vaccine including name manufacturer type of vaccine number of shots how it is given and links to ingredient information.
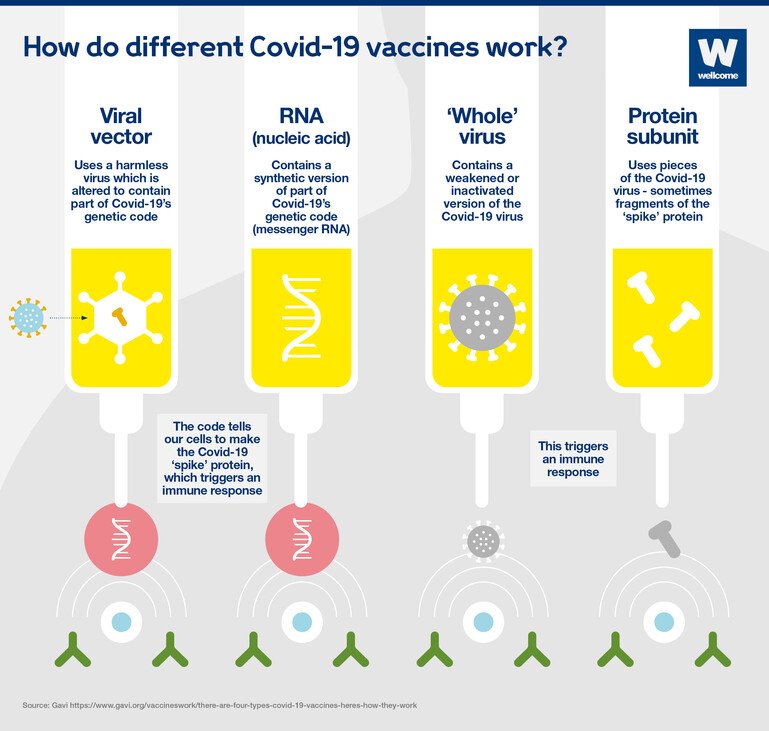
Once sufficient knowledge on the immunogenicity response to COVID-19 vaccines is acquired non-inferiority immunogenicity trialscomparing the immune response of a vaccine candidate to that of an authorised vaccinewould probably be the most common trial design. The present article uses clinical epidemiologic tools to critically appraise reports of efficacy in PfzierBioNTech and Moderna COVID-19 mRNA vaccine clinical trials. Protein based inactivated vaccines protein subunit VLP and T-cell based vaccines gene based DNA or RNA vaccines replicating or non-replicating viralbacterial vectored vaccines and a combination of both protein-based and gene-based live-attenuated virus vaccines. Clinical Trial of Recombinant Novel Coronavirus Vaccine Adenovirus Type 5 Vector Against COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Different types of immune responses are often measured including antibodies and cell-mediated immunity but phase II trials do not assess how well a vaccine actually works. Absolute risk reduction 07.