
Johnson And Johnson Covid Vaccine Effectiveness Cdc. HHS announced a plan to begin offering COVID-19 vaccine booster shots this fall. For more information see Updated. In fact the JJ vaccine was 93 percent effective at preventing hospitalization for COVID-19 14 days after vaccination and 100 percent effective 28 days after. A nurse administers the Johnson Johnson COVID-19 vaccine to a person at a neighborhood outreach occasion in Los Angeles COVID-19 vaccine effectiveness dropped to 66 against delta CDC finds Tech Reader Digest.
The clinical trial is the first real-world test of the vaccines efficacy against the highly transmissible variant. HHS announced a plan to begin offering COVID-19 vaccine booster shots this fall. People had the most protection 2 weeks after getting vaccinated. CDCs independent advisory committee the Advisory Committee on Immunization Practices will continue to meet and discuss data on the evolution of the pandemic and the use of COVID-19 vaccines. CDC uses several methods to evaluate COVID-19 vaccine effectiveness. CDC and FDA have recommended that use of JJJanssen COVID-19 Vaccine resume in the United States effective April 23 2021.
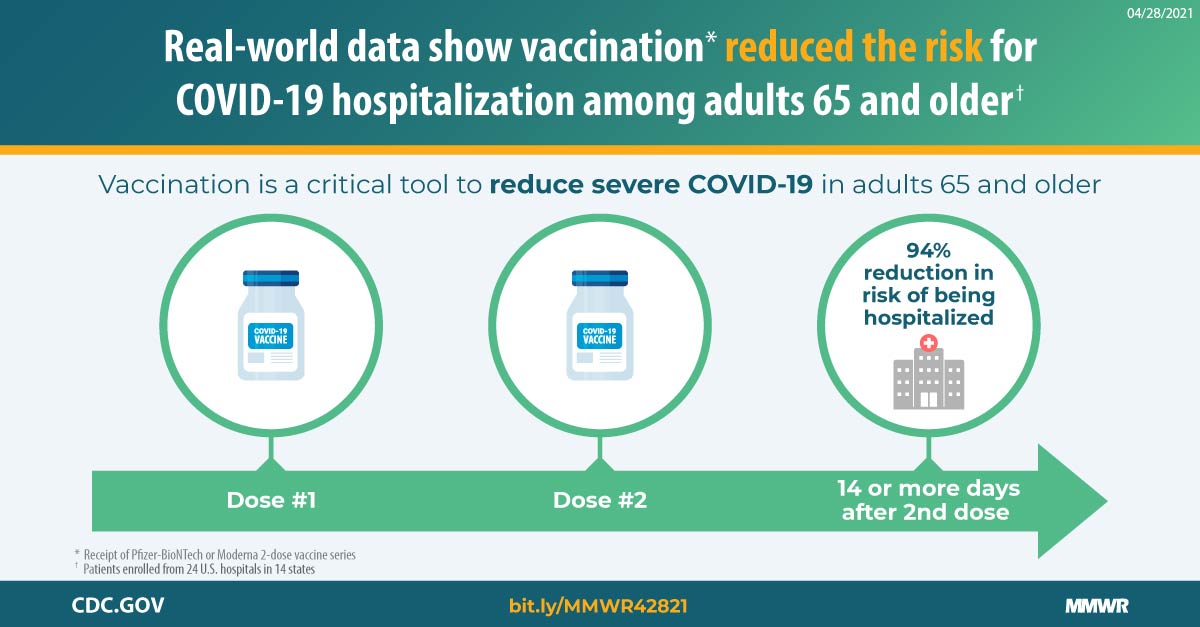
CDC has established methods to monitor vaccine effectiveness in real-world conditions and address ongoing and future questions around how vaccines perform.
A nurse administers the Johnson Johnson COVID-19 vaccine to a person at a neighborhood outreach occasion in Los Angeles COVID-19 vaccine effectiveness dropped to 66 against delta CDC finds Tech Reader Digest. The JJJanssen COVID-19 Vaccine was 663 effective in clinical trials efficacy at preventing laboratory-confirmed COVID-19 infection in people who received the vaccine and had no evidence of being previously infected. Among adults aged 75 years effectiveness of full vaccination for preventing hospitalization was 91 for Pfizer-BioNTech 96 for Moderna and 85 for Janssen COVID-19 vaccines. A review of all available data at this time shows that the JJJanssen COVID-19 Vaccines known and potential benefits outweigh its known and potential risks for those recommended to receive it. Food and Drug Administration and the US. CDCs independent advisory committee the Advisory Committee on Immunization Practices will continue to meet and discuss data on the evolution of the pandemic and the use of COVID-19 vaccines.