
How To Enroll In Covid Vaccine Trial. Parents with children ages 6 months to 11 years old listen up. Last week I got an offer to enroll my 14-month-old in a COVID vaccine trial for children writes Irin Carmon. You must be legally authorized in your jurisdiction to administer vaccines. On November 18 Pfizer and BioNTech announced that after conducting the primary efficacy analysis their mRNA-based COVID-19 vaccine met all of the studys.
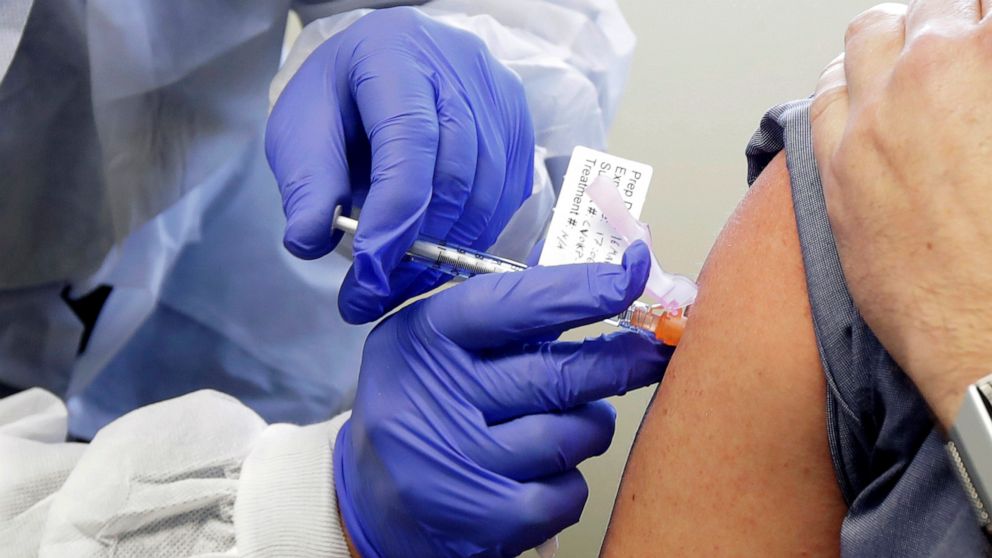
Is It Safe to Enroll Your Kid in a COVID Vaccine Trial. You and your child will be asked to return to the study site up to six. In the case of the Covid-19 vaccine trials this would include tests to make sure the prospective volunteer hasnt already been exposed to the virus which would make them ineligible. Taliban Drags 21-Year-Old Woman Out. You must be legally authorized in your jurisdiction to administer vaccines. Interested parties must complete a brief study and a.
Would you enroll your kid for Covid vaccine trial.
In this region Moderna is recruiting participants for a site at the Childrens Hospital of Philadelphia. Twelve to 20 weeks following their initial vaccination regimen participants will receive a single booster dose of the Moderna COVID-19 vaccine as part of the trial. To be a vaccination provider your health system or you as an independent provider are required to sign the CDC COVID-19 Vaccination Program Provider Agreement. PITTSBURGH The University of Pittsburgh School of Medicine through the Pittsburgh Vaccine Trials Unit PVTU is one of twelve sites across the country selected to participate in a National Institute of Allergy and Infectious Diseases clinical trial in which fully vaccinated adults will receive a third booster dose of a COVID-19 vaccine. This trial began July 27 2020 and completed enrollment of 46331 participants in January 2021. The Phase 3 clinical trial was designed to determine if the Pfizer-BioNTech COVID-19 vaccine is safe and effective in preventing COVID-19 disease.