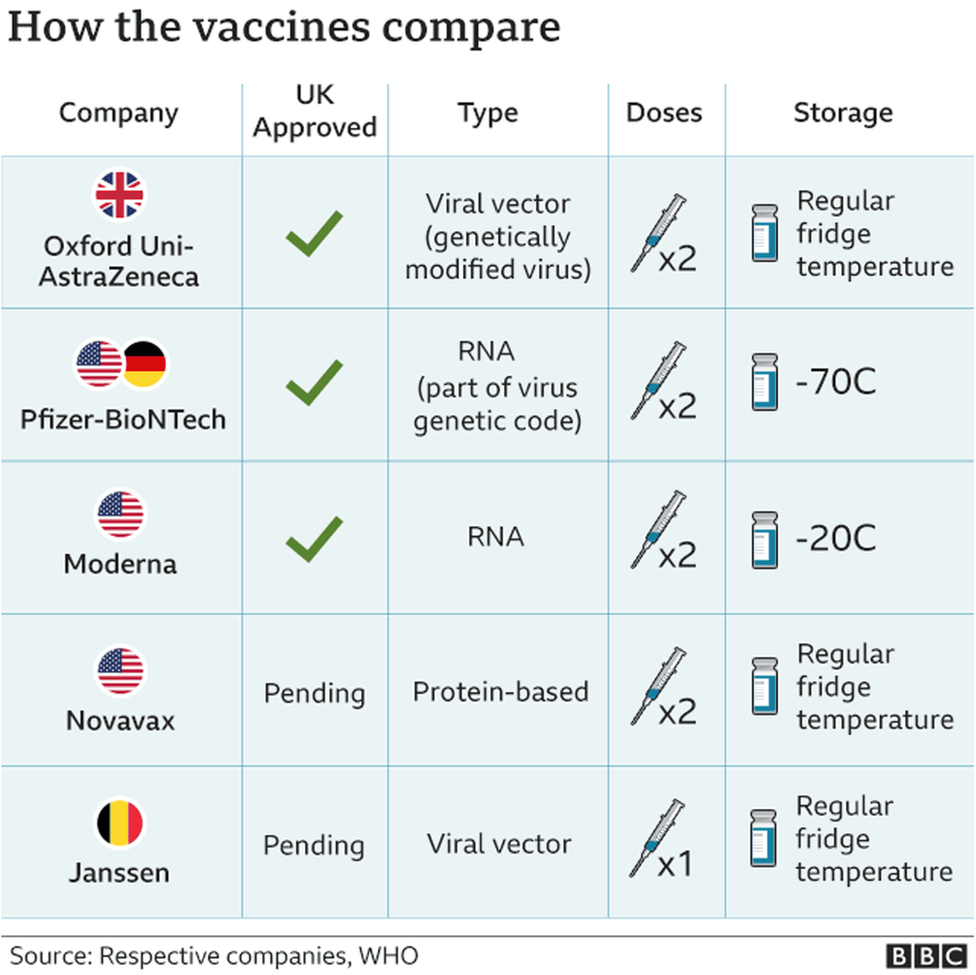
How Much Longer For A Covid Vaccine. Historically the timelines for bringing vaccines to bear on other pathogens show a much longer arc than 18 months. Theres a number of reasons people are hesitant to get the COVID-19 vaccine - many of them are of dubious validity including the notion that one has to pay for it. Tice said full review requires companies to. The EUAs for COVID-19 vaccines were granted based on an average of 2 months of safety follow-up data.
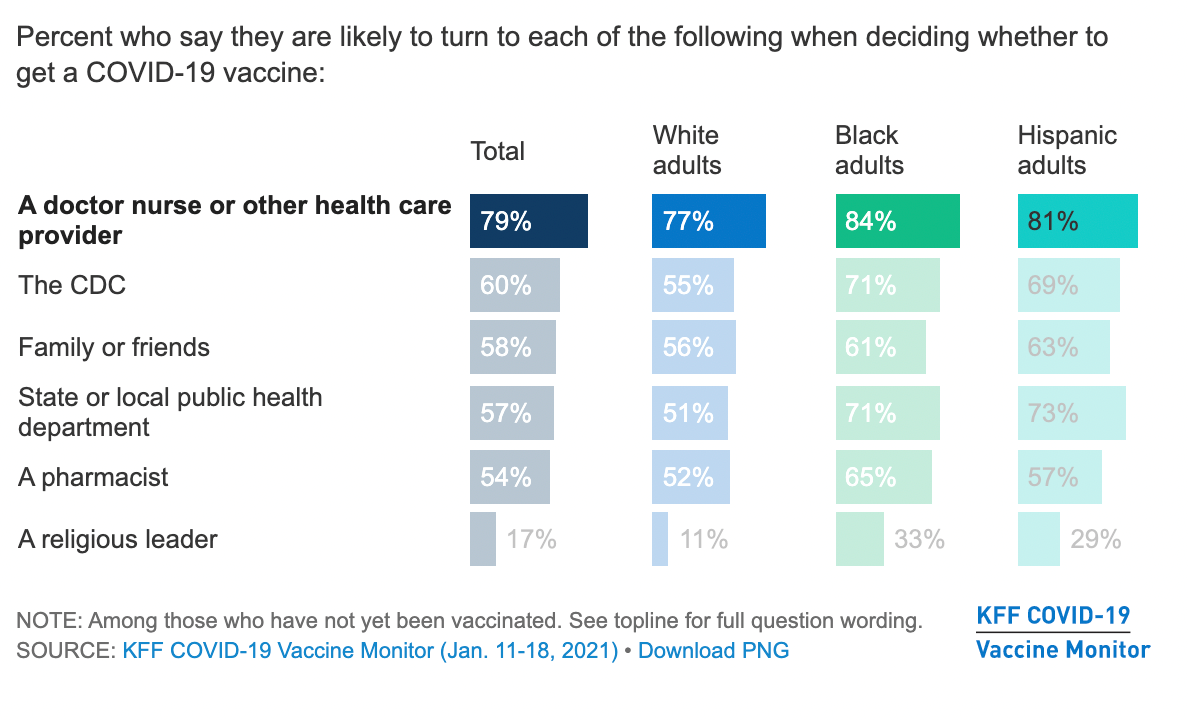
A typical vaccine development timeline takes 5 to 10 years and sometimes longer to assess whether the vaccine is safe and efficacious in clinical trials complete the regulatory approval processes and manufacture sufficient quantity of vaccine doses for widespread distribution. The programme which plans to offer tests to thousands of adults per day aims to improve our understanding of how much protection antibodies give us following covid-19 infection and vaccination. The timeline for a COVID-19 vaccine to be approved for children under the age of 12 was initially set for fall 2021. None of the vaccines has been formally approved and only one COVID. We do not yet know how many of these patients will experience long COVID but its estimated that at least 7 experience extended symptoms says co-author Anthony Komaroff MD the Steven P. From the perspective of advocates of a great reset globally coordinated covid-19 vaccination underscores the need for global structures and organizations that can then be used for other global purposes such as effectively.
11 2021 in Long.
Take action on UpLink Experts dont know yet how long COVID-19 vaccines will be effective. Tice said full review requires companies to. 11 2021 in Long. The programme which plans to offer tests to thousands of adults per day aims to improve our understanding of how much protection antibodies give us following covid-19 infection and vaccination. Theres a number of reasons people are hesitant to get the COVID-19 vaccine - many of them are of dubious validity including the notion that one has to pay for it. The timeline for a COVID-19 vaccine to be approved for children under the age of 12 was initially set for fall 2021.