How Many Covid Vaccines In Clinical Trials. As many as. In just four months the COVID-19 vaccines have killed more people than all available vaccines combined from mid-1997 until the end of 2013 a period of 155 years. It is planned that the study will be conducted with two separate cohorts. A Facebook post states that the Covid-19 vaccines will not have finished testing until 2023.
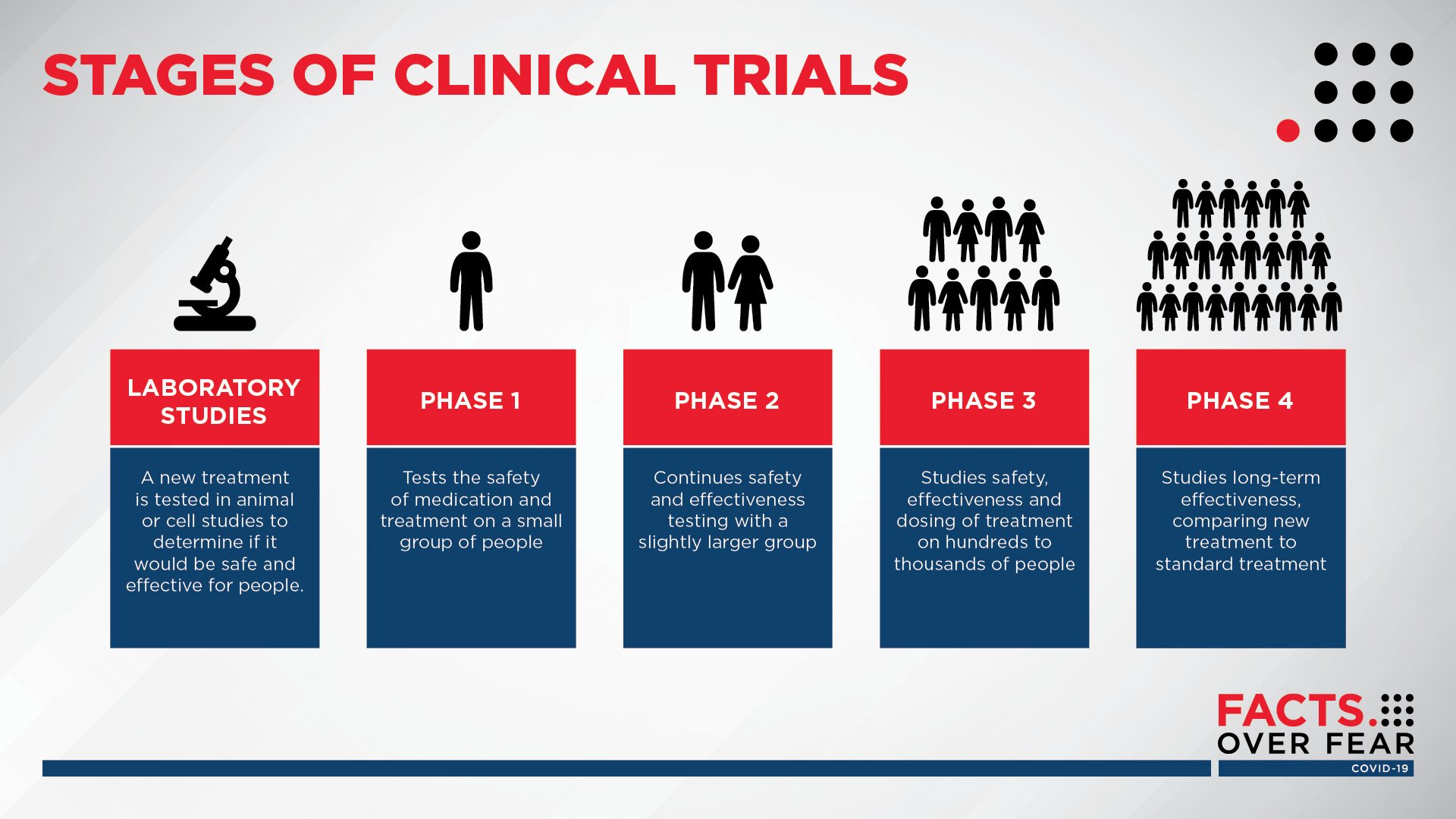
Clinical trials are medical research studies to test new ways to prevent detect or treat diseases. 999 Clinical trial phase. 104 rows The Vaccines and Related Biological Products Advisory Committee will meet in open session to discuss Emergency Use Authorization EUA of the Janssen Biotech Inc. Participants of clinical trial of Covid-19 vaccine can download fully-vaccinated certificate. 16 and older Number of people all ages. In particular the post states that studies on the three vaccines.
The experimental vaccine and placebo were both manufactured by Sinovac Research Development Co Ltd.
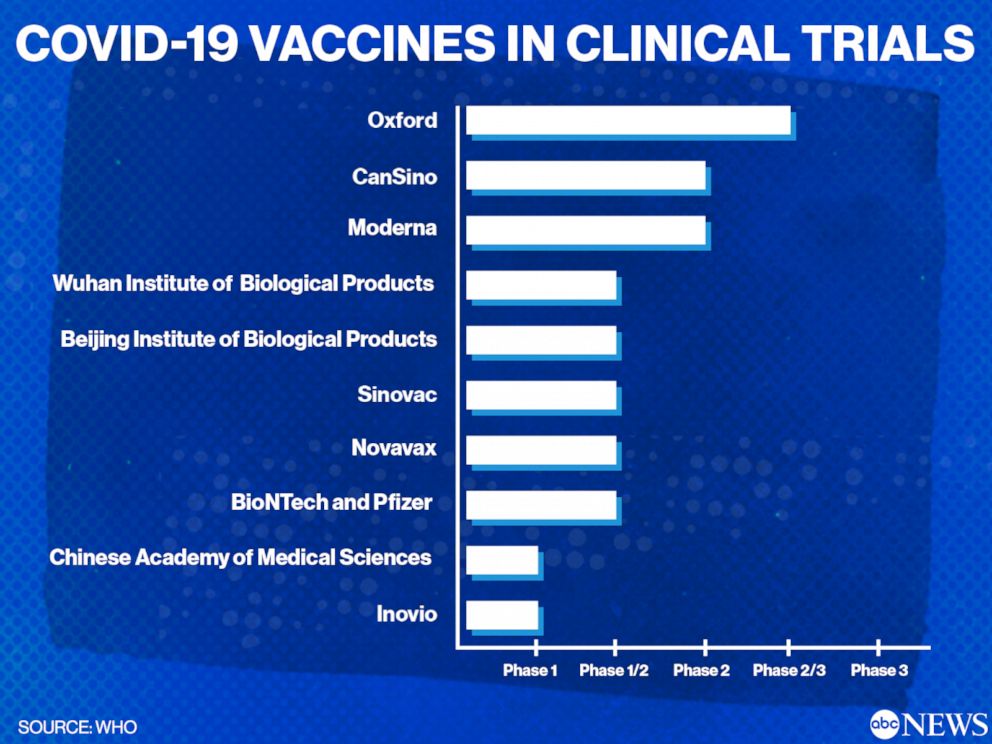
COVID-19 vaccine clinical trials including vaccines in earlier stages of development by visiting clinicaltrialsgov. Clinical trials for COVID-19 vaccines were carried out before they were approved by governments and rolled-out to the public. It is planned that the study will be conducted with two separate cohorts. AstraZeneca COVID-19 vaccine Novavax COVID-19 vaccine Learn more about US. 1 of 3 claims. COVID-19 Clinical Trials.