
How Many Covid 19 Vaccines Are In Clinical Trials. Among them 63 vaccines have been approved for clinical trials and 27 are evaluated in phase 3 clinical trials. Phase 1 and 2a clinical trials normally last several months to even a year before proceeding to Phase 2b or Phase 3 trials in which the pool of people receiving the vaccine increases. Pfizers trial enrolled over. No trial phases have been skipped.
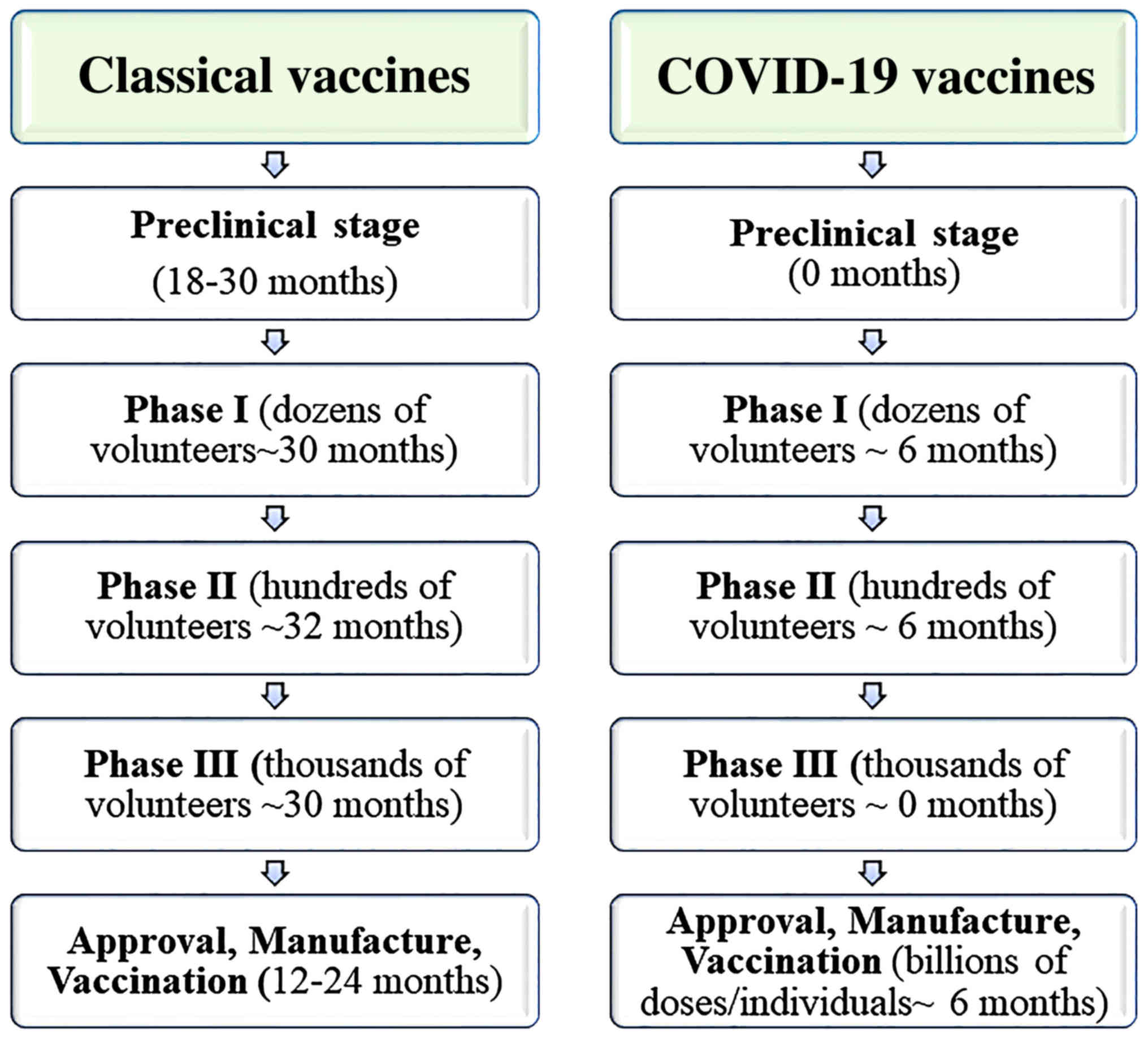
The Pfizer vaccine Comirnaty has been assessed in global studies across three phases. Third doses of covid-19 vaccines will be rolled out to. As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States. But because there is an urgent need for COVID-19 vaccines and the FDAs vaccine approval process can take months to years the FDA first gave emergency use authorization to COVID-19 vaccines based on less data than is. We hope to build upon our experience in COVID-19 vaccine clinical trials to partner on this new and innovative vaccine technology he said. Clinical trials for COVID-19 vaccines were carried out before they were approved by governments and rolled-out to the public.
Clinical trials for a COVID-19 vaccine are designed to assess the safety and efficacy of the vaccine.
We hope to build upon our experience in COVID-19 vaccine clinical trials to partner on this new and innovative vaccine technology he said. A vaccine may be licensed for use after a successful Phase 3 trial. Information about the JJJanssen COVID-19 vaccine including name manufacturer type of vaccine number of shots how it is given and links to ingredient information. Clinical trials for COVID-19 vaccines were carried out before they were approved by governments and rolled-out to the public. Learn about safety data efficacy and clinical trial demographics. Issuance of Emergency Use.