
How Many Clinical Trials For Covid 19 Vaccine. Johnson Johnson just announced the launch of a Phase 3 clinical trial for its COVID-19 vaccine candidate. Up to now 237 candidate vaccines against SARS-CoV-2 are in development worldwide of which 63 have been approved for clinical trials and 27 are evaluated in phase 3 clinical trials. For example the Moderna vaccine clinical trial protocol works on the assumption that one out of 133 people will develop symptomatic COVID-19 over a six-month period. In total these trials seek to enrol 280000 people from 34 countries.
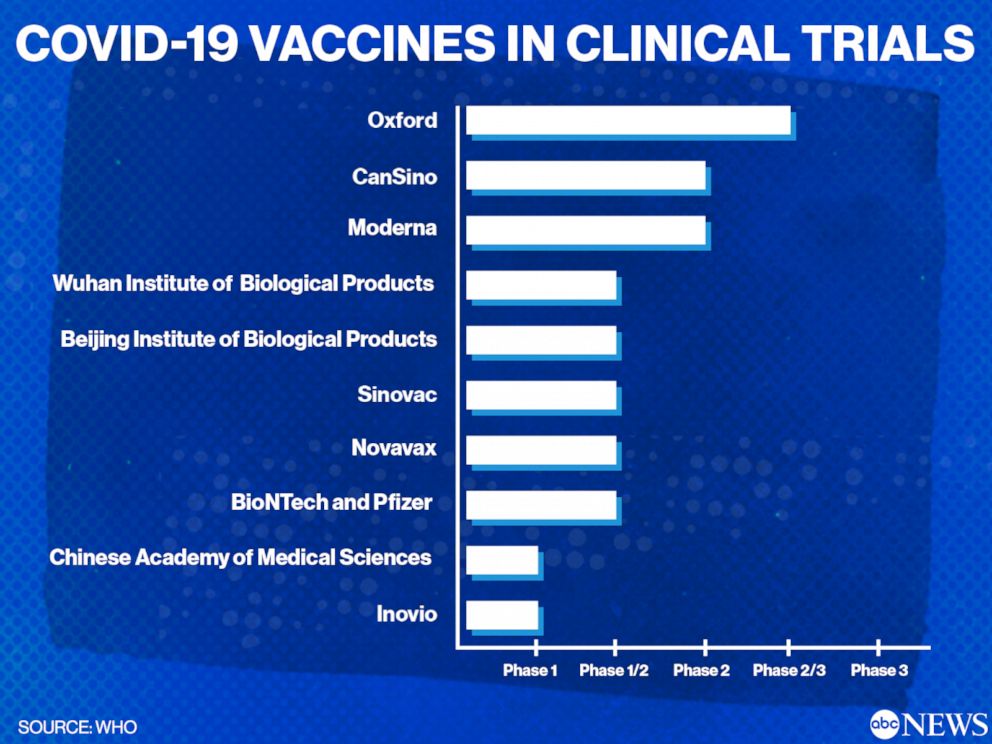
Third doses of covid-19 vaccines. The data collection and analysis are ongoing in order to allow up to two years of follow up on participants. The effect will be particularly acute in high-income countries where the entire adult or older population could be vaccinated by late 2021. Phase I clinical trials are the first step in assessing vaccines in people. Tedros Ghebreyesus the Director-General World Health Organisation WHO says a number of vaccines are now in phase three clinical trials to. In total these trials seek to enrol 280000 people from 34 countries.
Sponsored by Aposave Visit Company.
For example the Moderna vaccine clinical trial protocol works on the assumption that one out of 133 people will develop symptomatic COVID-19 over a six-month period. However for Covid-19 vaccines some of those phases were combined to hasten the process. Up to now 237 candidate vaccines against SARS-CoV-2 are in development worldwide of which 63 have been approved for clinical trials and 27 are evaluated in phase 3 clinical trials. In normal circumstances each step can take two years or more to complete. The effect will be particularly acute in high-income countries where the entire adult or older population could be vaccinated by late 2021. Johnson Johnson just announced the launch of a Phase 3 clinical trial for its COVID-19 vaccine candidate.