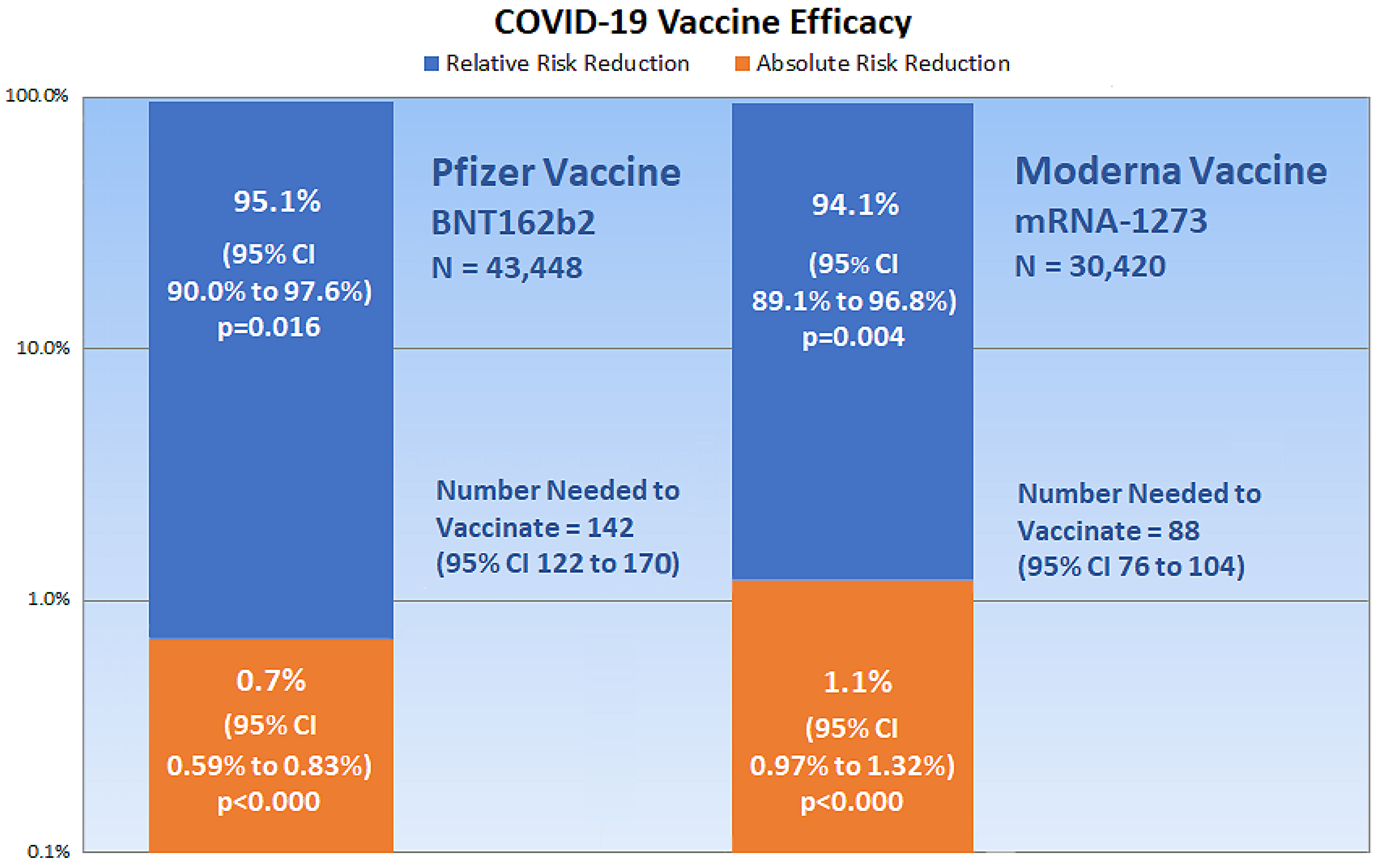
Covid Vaccine Trial Outcomes. A 3rd shot of Pfizer-BioNTechs Covid vaccine sharply will increase the degrees of antibodies towards the coronavirus the businesses reported on Wednesday. Covid-19 vaccine trials are currently designed to tabulate final efficacy results once 150 to 160 trial participants develop symptomatic covid-19and most trials have specified at least one interim analysis allowing for the trials to end with even fewer data accrued. Results of the study. Almost everyone experiences arm pain at the injection site.
The businesses have been saying the outcomes of a examine of 306 volunteers who acquired a booster. Many of the secondary outcome measures involve examining or measuring samples from participants up. Covid-19 vaccine trials are currently designed to tabulate final efficacy results once 150 to 160 trial participants develop symptomatic covid-19and most trials have specified at least one interim analysis allowing for the trials to end with even fewer data accrued. Other symptoms can include low grade fever body ache chills fatigue and headache. Preliminary findings did not show obvious safety signals among pregnant persons who received mRNA Covid-19 vaccines. Based on results from the clinical trial the vaccine was 91 effective in preventing COVID-19 disease.
Rigorous studies of these vaccines in action are an urgent priority globally Post-introduction vaccine studies provide practitioners and policy makers with the kind of evidence that clinical trials cannotincluding real world vaccine effectiveness against multiple clinical outcomes.
They counted how many pregnancies resulted in live births miscarriages preterm births and congenital anomalies. Rigorous studies of these vaccines in action are an urgent priority globally Post-introduction vaccine studies provide practitioners and policy makers with the kind of evidence that clinical trials cannotincluding real world vaccine effectiveness against multiple clinical outcomes. VOVVN - A local research team working on developing Nano Covax a homegrown COVID-19 vaccine have unveiled the promising outcomes of the mid-term phase 3a clinical trials. No adverse pregnancy-related outcomes including adverse outcomes that affected the infant were associated with vaccination in these trials. However more longitudinal follow-up. The assessment found that COVID-19 symptomatic illness was reduced by 94 among HCP who were fully vaccinated defined in this study as seven or more days after receipt of a second vaccine dose and by 82 among those who were partially vaccinated defined in this study as 14 days after receipt of dose one through six days after dose two.