Astrazeneca Covid Vaccine Phase 2 Results. The preliminary results of this first-in-human clinical trial supported clinical development progression into ongoing phase 2 and 3 trials the researchers concluded in Safety and immunogenicity. We do not know if COVID-19 Vaccine AstraZeneca is as effective in people with immunocompromise compared with the rest of the population. Characteristics of AZD1222 vaccine against COVID-19. Results from a phase 2 randomised trial of one injection of non-replicating adenovirus-vectored COVID-19 vaccine.

COVID-19 Vaccine AstraZeneca also known as AZD1222 or ChAdOx1S recombinant was - developed by Oxford University United Kingdom and AstraZeneca and is a replication-deficient chimpanzee adenovirus-vectored vaccine expressing the full-length SARS CoV-2 spike glycoprotein gene. The manufacturers said the vaccine was undergoing comprehensive phase 3 trials to confirm the results. According to the study results a single dose of AZD1222 led to a four-fold increase in antibodies to the SARS-CoV-2 virus spike protein in 95 of subjects one month following vaccination. Severe or critical COVID-19 was defined as having SARS-CoV-2. This is a step in the right direction. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2.
They also highlight the need for further studies of vaccine efficacy in.
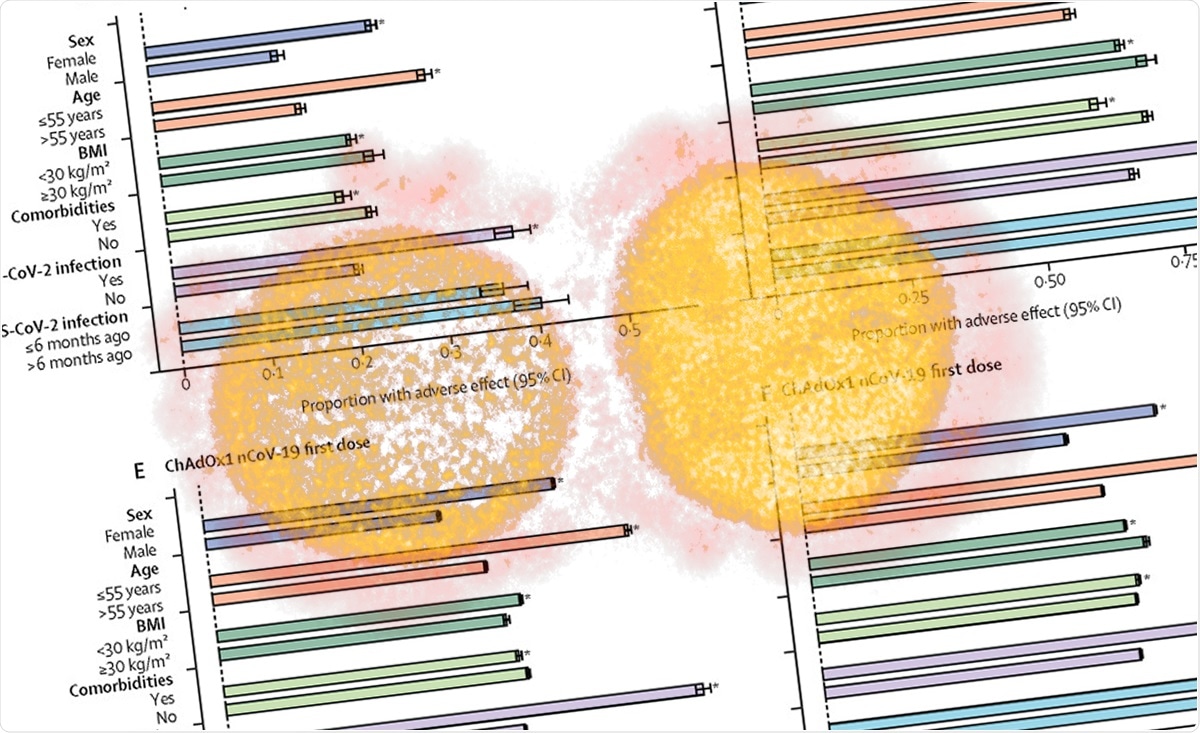
The manufacturers of Oxford-AstraZeneca coronavirus vaccine have said that it produced fewer side effects in people aged 56 and over in phase 2 trial results. The next stage of. A clinical trial is being conducted of COVID-19 Vaccine AstraZeneca given to people with stable HIV infection with results expected in a few months. Furthermore a T-cell response that peaked by day 14 and maintained two months after injection was observed in all participants. According to the study results a single dose of AZD1222 led to a four-fold increase in antibodies to the SARS-CoV-2 virus spike protein in 95 of subjects one month following vaccination. Developed by Oxford University and AstraZeneca it is given by intramuscular injection using as a vector the modified chimpanzee adenovirus ChAdOx1.