
Astrazeneca Covid 19 Vaccine Clinical Trial Results. We report on the first clinical efficacy results of ChAdOx1 nCoV-19 in a pooled analysis of phase 23 trials in the UK and Brazil and safety data from more than 20 000 participants enrolled across four clinical trials in the UK Brazil and South Africa. Data are first Phase 3 trial results of a coronavirus vaccine to be published in peer-review literature Today University of Oxford and AstraZeneca researchers present a pooled analysis of Phase 3 trials of a vaccine against SARS-CoV-2 across two different dose regimens resulting in an average efficacy of 704. SeventyFour iStock The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. New vaccine efficacy results are reported now in The Lancet.
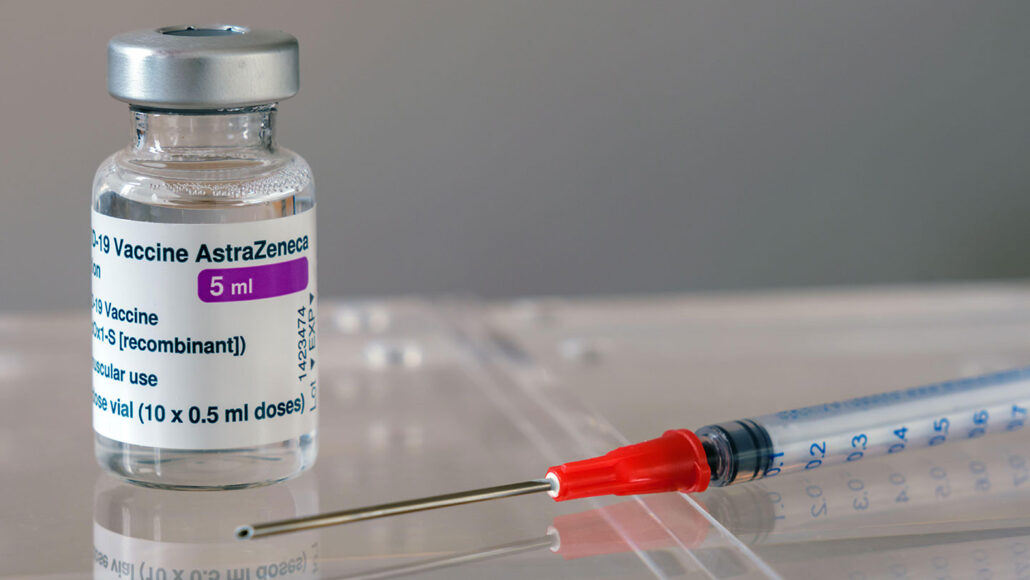
The high-level results from the phase 3 clinical trial called PROVENT were unveiled today. New vaccine efficacy results are reported now in The Lancet. The drug made from a combination of two antibodies was initially developed as a treatment for those who had already been exposed to the disease. Studies carried out in 2020 showed that the efficacy of the. We report on the first clinical efficacy results of ChAdOx1 nCoV-19 in a pooled analysis of phase 23 trials in the UK and Brazil and safety data from more than 20 000 participants enrolled across four clinical trials in the UK Brazil and South Africa. Results from a large US clinical trial involving more than 32000 people and testing the AstraZenecaOxford COVID-19 vaccine candidate are finally available -.
The results from a trial that looked at how AstraZeneca and Sputnik Light doses reacted when combined.
The results from a trial that looked at how AstraZeneca and Sputnik Light doses reacted when combined. The COVID-19 pandemic has caused major disruption to healthcare systems with significant socioeconomic impacts. The PROVENT pre-exposure prophylaxis trial showed AZD7442 could potentially provide long-lasting protection with demonstrated clinical trial success. We report on the first clinical efficacy results of ChAdOx1 nCoV-19 in a pooled analysis of phase 23 trials in the UK and Brazil and safety data from more than 20 000 participants enrolled across four clinical trials in the UK Brazil and South Africa. Representational image Drug firm AstraZeneca on Friday announced positive results from a trial of a treatment for COVID-19 symptoms. The drug was made from a combination of two antibodies which was initially developed as a treatment for those who had already been exposed to the virus.